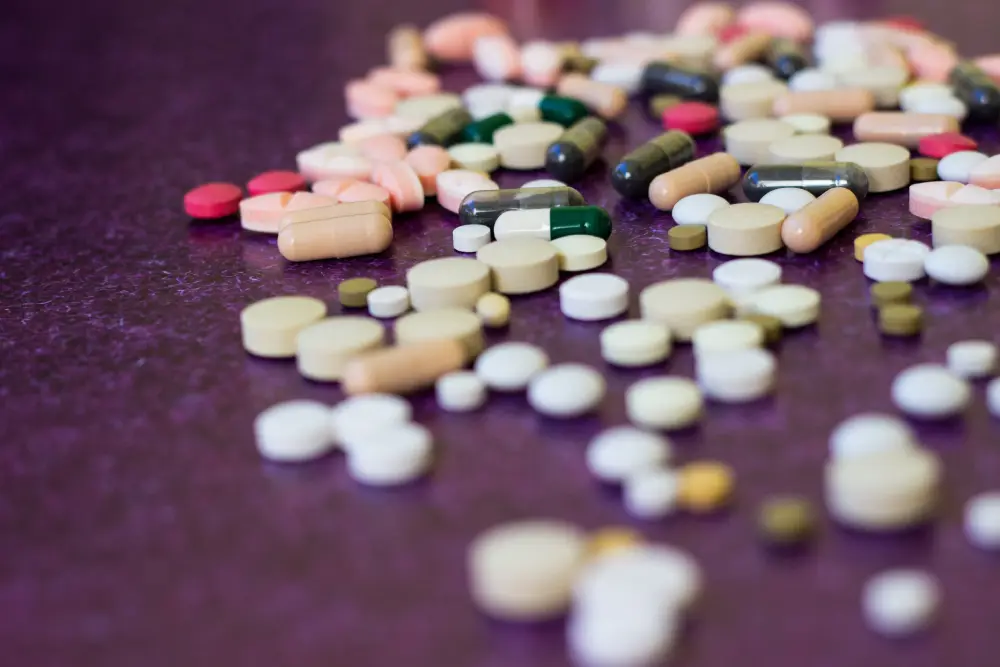
Some of the most common questions about DEA controlled substance management and disposal are below:
- EMERGENCY RESPONSE 800-966-9282
- CUSTOMER SERVICE 888-834-9697
- Services
- Waste Management & Disposal
- Waste Disposal
- Hazardous Waste Removal & Disposal
- Waste Recycling & Sustainability
- DEA Controlled Substance Management & Disposal
- Onsite Environmental Services
- Environmental Remediation Support Services
- Lab Pack
- HPLC Solvent Waste Collection
- Waste Compliance, Analytics & Reporting
- Cylinder Management
- EHS Services for Cannabis Enterprises
- EHS Consulting services
- EHSOne: Onsite EHS Managed Services
- EHS Operational Support & Staff Augmentation
- EHSGreen: Innovative Sustainability & Recycling Services
- Lab Sustainability
- EHS Compliance Reporting
- EHS Plans & Permits
- EHS Audits
- Biosafety Consulting & Services
- Joint Commission Consulting
- Perimeter Air Monitoring
- Industrial Hygiene
- Lab & facility Services
- EHSLearn
- Waste Management & Disposal
- Industries
- About
- Resources
- Service Locations
- Contact